Liquid crystal phases
Liquid crystal phases have a range of different structures, but all have one thing in common: they flow similarly to viscous liquids, but show the physical behavior of crystals.
A crystal that flows?
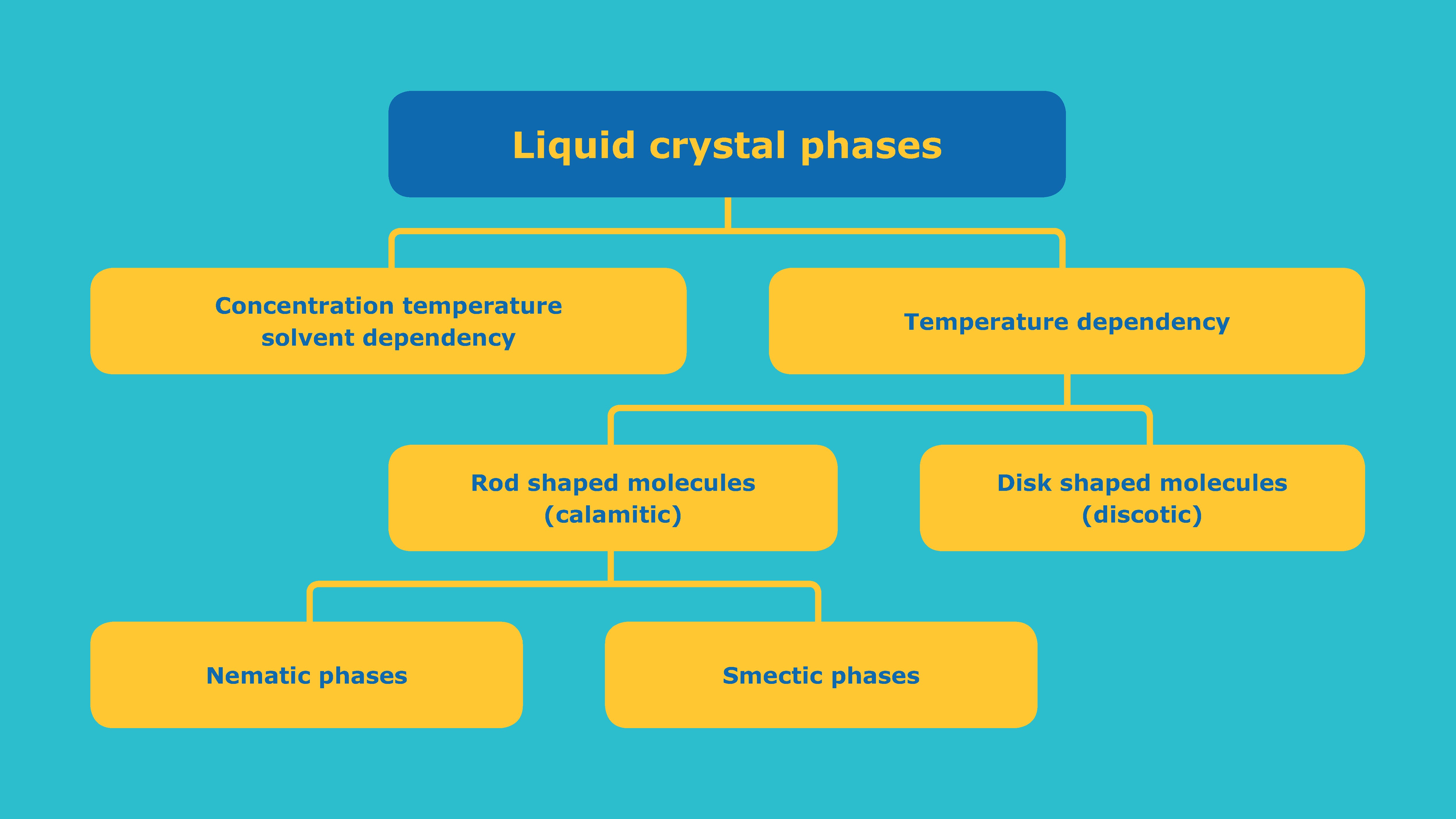
Since the discovery of liquid crystalline phase in 1888, a large number of different liquid crystal phases has been discovered. Although different liquid crystals strongly vary in chemical structure they all have one thing in common: they flow in a similar way to viscous liquids but show anisotropic physical properties of crystals. Their appearance depends on various criteria such as the molecular structure, the temperature as well as their concentration and the solvent.
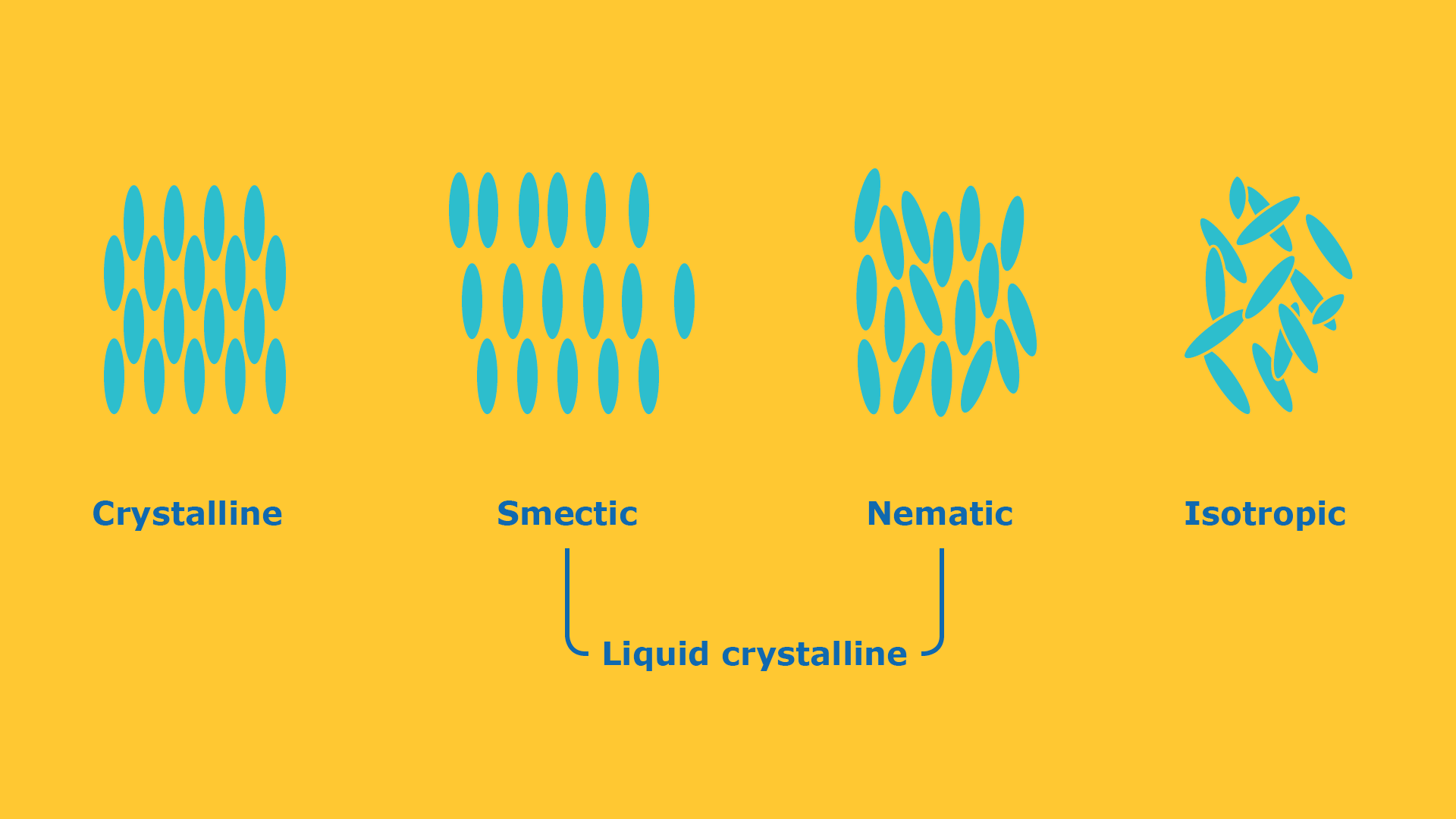
A crystal is characterized by a three-dimensional long range orientational- and positional order. This long-range order results in three-dimensional anisotropic physical properties. Isotropic liquids only show short range order and are thus characterized by isotropic physical properties.
Liquid crystals consist of anisotropic molecules and show a degree of order between the crystal and the isotropic phase. The least ordered liquid crystal phase only exhibits a one-dimensional long range orientational order. This is the so-called nematic phase. In smectic phases the anisotropic molecules are additionally oriented in layers and consequently their degree or order is between the crystal phase and the nematic phase. Liquid crystals are therefore also characterized by anisotropic physical properties.
The degree of order has a direct correlation to the viscosity of the material. Nematic liquid crystals almost flow like an isotropic liquid while smectic liquid crystals behave like a highly viscous liquid.
In LCD technology, the thermotropic nematic phase is by far the most significant phase. It is formed from road shaped calamitic molecules and exhibits a one-dimensional long range orientational order, which is represented by the director. The director can be switched by electric fields in an LCD and thus the liquid crystals act as a light valve.
As the temperature rises, the order of a system decreases. The temperature at which a liquid crystal phase is converted to the isotropic liquid is called the clearing point. A substance may form one or more liquid crystal phases if the structural conditions allow this. However, the appearance of liquid crystal phases is not necessarily a consequence of the molecular structure.

Ferroelectric Nematics
A new LC phase experimentally proven after 100 years of its theoretical prediction - The next revolution in electronics?
Extraordinary properties could enable exciting new applications
Nematic liquid crystals display a unique mix of fluid- and solid-like behaviors, and the term nematic derives from the Greek νεμα, which reads “nema” and means “thread” – a reference to the characteristic thread-like topological defects they contain. These molecules are polar, with one end carrying a positive charge and the other a negative one. In a traditional nematic crystal, half of these molecules will on average point in one direction and the other half in the other.
The “ferroelectric nematic” (NF) phase is a more organized new phase of matter in liquid crystals, first predicted to exist over 100 years ago and first hypothesized in the 1910s by the Nobel laureates Peter Debye and Max Born with first experimental proof published in 2017 (Kikuchi, Mandle et al.).
Within specific patches or “domains”, all of its molecules point in the same direction. This phenomenon is known as polar ordering. They predicted that if a liquid crystal were designed correctly, its molecules could spontaneously fall into a polar ordered state.
Shortly afterwards, researchers discovered solid crystals in which molecules did indeed point in uniform directions. The direction of these molecules could be reversed, from right to left or vice versa, when an electric field was applied – a property that inspired the name “ferroelectrics” because of similarities to ferromagnets. Despite much searching, however, a liquid crystal phase that behaves in the same way proved elusive – until now.
The new nematic phase was discovered having remarkable properties, and it has been suggested that this is the long sought-after NF phase. This has the potential to be a hugely significant discovery from both fundamental and technological viewpoints. The polar ordering in the NF phase could make it vastly more sensitive to an electric field than the conventional N phase, and this might dramatically improve the performance of liquid crystal display devices in terms of both speed and power consumption. In addition, this new phase has the potential of generating transformative new fundamental chemistry, physics and biology and could open up a wealth of technological innovations.
Facts & figures
The Ferroelectric Nematics Application Challenge
In 2022 Merck KGaA, Darmstadt, Germany hosts the Ferroelectric Nematics Challenge, an application competition to identify the best potential applications for ferroelectric nematic LCs: by technical approach and business impact.
Work with us!