»The birth of a cancer drug. In 1980, Dr. John Mendelsohn envisioned a new cancer treatment so radical he could not get U.S. government funding. Now, after years of fund-raising and research, his drug could become a blockbuster. Here is the story of C225.«
Business Week, European Edition July 30, 2001
In the mid-1980s, the company develops active ingredients to treat solid tumors. Collaboration with the Wistar Institute in Philadelphia very soon results in strategies for the immunological therapy of tumors based on antibodies directed again stepidermal growth factor receptor (EGFR) and specific glycolipids on the surface of tumor cells. Alliances with leading research institutes in the United States and Germany generate new insights into potential targets.
Merck KGaA, Darmstadt, Germany, has the necessary expertise in medicinal chemistry and in the manufacture and characterization of monoclonal antibodies, in particular with the support of an alliance with the Medical Research Council in Mill Hill, United Kingdom. Molecular biological laboratories at the company initially focus their attention on antibody technology projects. Three approaches in immunology research are investigated: monoclonal antibody therapy, active immunization, and preventing metastasis via adhesion receptors. The company investigates compounds in collaboration with Scripps Clinic in San Diego, California.
Two company acquisitions enable expansion of the research effort. U.S.-based Lexigen is acquired in 1998 specifically to secure a patent position for immuno-cytokine technology. One of Lexigen’s founders was the immunologist and Nobel laureate Susumu Tonegawa. In the year 2000, the company acquires Biovation, a highly specialized Scottish company working on the innovative approach of antibody deimmunization.
The lion’s share of the € 577 million Merck KGaA, Darmstadt, Germany, spends on research and development in 2001 goes to the Pharma business to advance oncology and above all biotherapeutic agents. Nine oncology drug candidates are in clinical trials.
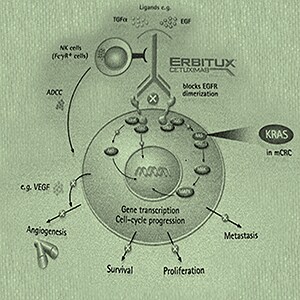
In 1998, Merck KGaA, Darmstadt, Germany, acquires licenses for C225/cetuximab from ImClone, New York. Cetuximab is a targeted therapy for the treatment of certain types of colorectal cancer and squamous cell carcinoma of the head and neck. This monoclonal antibody acts by binding and blocking epidermal growth factor receptor (EGFR) in a highly effective and targeted manner. EGFR molecules are expressed on the cell surface of many tumors, and promote cell growth and division when stimulated by growth factors. Cetuximab blocks these receptors, thus interfering with tumor growth.
The drug was first approved under the name Erbitux in Switzerland in 2003, then in 2004 in the United States and the European Union.
Prior to the 1980s, the company had some involvement in the field of oncology as a supplier of adjuvant therapy products such as restorative agents, painkillers and corticoids. The Oncology/Immunology franchise is officially positioned in the Pharmaceuticals business sector in 1987.
Merck KGaA, Darmstadt, Germany, is adept both at genome research and assessing the associated data using bioinformatics. By teaming up with an external partner, the aim was thus to create a competitive advantage in biological validation of target molecules in cell culture experiments, immunohistological analysis and efficacy in animal testing models.
In the early 1980s, the physician John Mendelsohn (left) and the biologist Gordon H. Sato had no idea about the role their invention would play in oncology one day. The two professors at the University of California sought to stop cancer cell growth by blocking the molecule that tells the cells to grow.