»A curious crystallized material but which does not seem to be ammonium cyanate«
Friedrich Wöhler, 1825
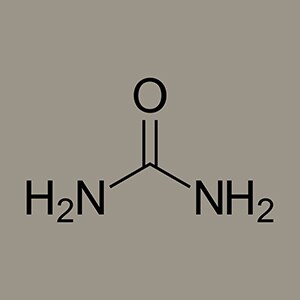
The true starting point of organic chemistry is not until 1827 when chemist Friedrich Wöhler synthesizes urea. Wöhler succeeds in producing urea by boiling down an aqueous solution of ammonium cyanate – an example of the synthesis of an organic compound (such as sugar or urea) from inorganic material, something hitherto thought impossible.
Vitalism, or the prevailing belief among scientists that an organic compound required a vital force, is thus disproved. This path-breaking urea is in fact the outcome of a failed experiment. Wöhler actually intends to synthesize ammonium cyanate (NH4OCN) but obtains urea (CO[NH2]2) instead of the expected inorganic salt. Without knowing it, Wöhler had already succeeded in synthesizing urea in 1825 by reacting cyanogen with liquid ammonia. However, he only recognized the urea as such in his experiment two years later, having previously described it as »a curious crystallized material«.
He reports his find to Jöns Jakob Berzelius, the Stockholm-based chemical scientist later known as the »father of modern chemistry«: »I must tell you that I can make urea without a kidney or even a living creature, be it man or dog […]. Can this artificial formation of urea be considered an example of the generation of an organic material from inorganic materials?«
Few of Wöhler’s contemporaries grasp the true meaning of this experiment. One of those intrigued by Wöhler’s discovery is Emanuel Merck, whose subsequent work is profoundly inspired by it. The very year Wöhler completes his groundbreaking studies, his friend Emanuel Merck embarks on large-scale isolation of alkaloids and the sale of standardized products, thus laying the foundation for »Chemische Fabrik E. Merck«. Wöhler in turn appreciates Emanuel Merck as a respected partner and source of highly purified Emanuel ingredients for his research. A little bowl of white urea crystals made by Wöhler which he gives to Emanuel Merck bears witness to the thriving scientific relationship between the two men. That little ounce of urea from organic chemistry’s first hour is a symbol of a paradigm shift in chemistry and testament to the role of Emanuel Merck in changing the direction of pharmaceutical research. It is treasured to this day as one of the most valuable pieces in the company Archives.
Uroscopy is a centuries-old diagnostic method. Coloration or sediment are thought to be symptoms of disease. In 1727, Dutch physician and chemist Herman Boerhaave describes »sal nativ usurinae«, or »natural salt of urine«. Even 100 years later, scientists still dispute the chemical composition of this »urea«.
The first synthesis and associated elucidation of urea’s atomic composition did not succeed until 1828 through the efforts of Friedrich Wöhler, depicted in the photo above with colleagues (from left) Johann Heinrich Buff, Friedrich Wöhler, Hermann Kopp and Justus von Liebig. The chemist Wöhler holds the pharmacist Emanuel Merck in high esteem. He gives him a dish containing urea as a present
In an exchange of letters by the scientific community, Wöhler’s work is an exciting topic since it disproves a dogma. The synthesis of urea from ammonium cyanate shows for the first time that compounds previously associated exclusively with living organisms can be generated from inorganic matter.