»It was like seeing everything through a yellow filter. Every single object, indoors or outdoors, was tinted yellow. The water streaming from the tap was yellow…«
A report by a patient on digitalis therapy in the journal Fortschritte der Medizin, 1971
In his book entitled »An Account of the Foxglove and Some of its Medical Uses« published in 1785, British doctor and botanist William Withering describes the use of digitalis to treat »dropsy«, the water retention typical of congestive heart failure. The digitalis effect is spectacular, but it is equally clear right away that the active ingredient amount necessary for diuresis and improved cardiac output and the toxic dose liable to produce vomiting and vision deficiencies are very similar indeed.
Effective yet safe treatment with foxglove preparations is not a realistic option until advances in the 19th century make it possible to isolate and test the active ingredients. Scientists will go on to spend decades developing a standardized dosage form for delivery of the »active components« from the highly toxic foxglove plant. It takes until 1874 to determine that the extract contains eight different cardiac glycosides; up to then, »digitalin« was administered in a random mixture of highly active but also highly toxic compounds.
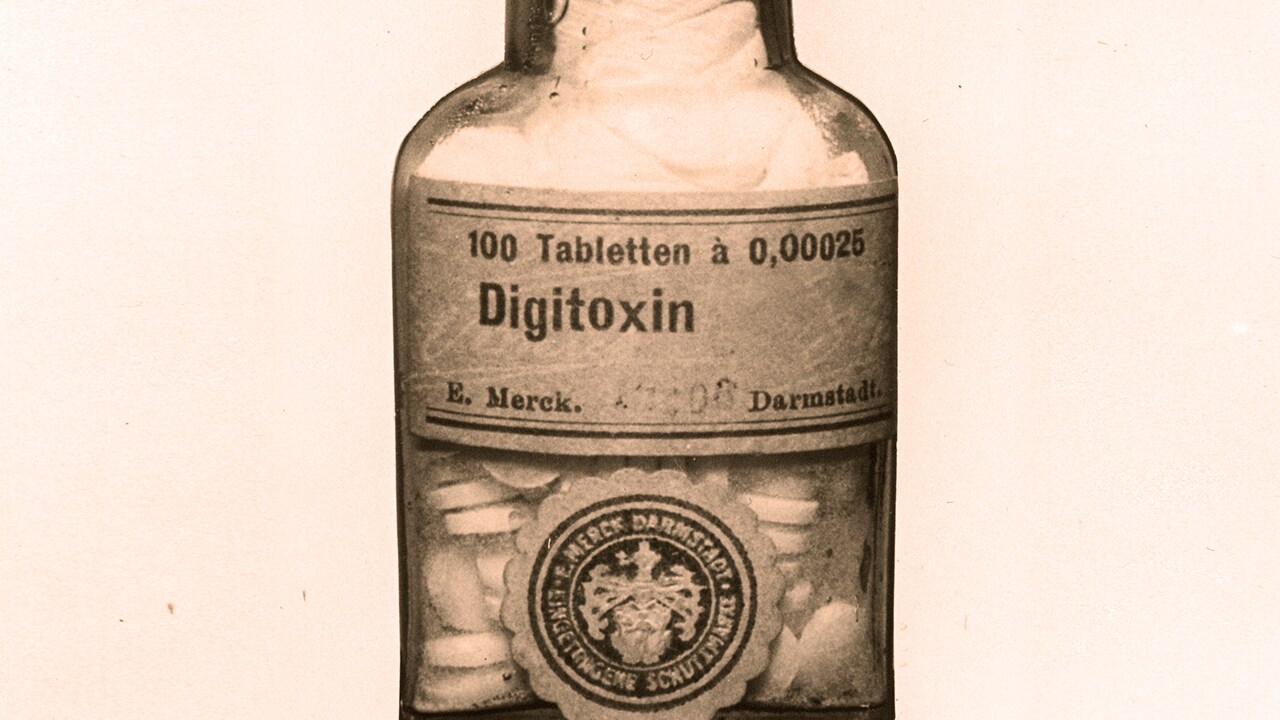
With his expertise in natural substance chemistry, the company soon recognizes the potential of this medicinal product. The 1857 price list includes »Digitalinum Purum« and »Digitaleinum by Schmiedeberg’s method«. The 1875 version features pure »Digitalinum crystallisatum«, which is named »Digitoxin«. The drug presents a challenge in terms of production. The annual report for 1896/97 reads: »Presenting these active components still poses numerous difficulties […]. 600k digitalis leaves were processed. The yield was only 66.9 g of digitoxinas two attempts to improve the method failed. «Then another problem literally comes into focus. The »colored light phenomena «associated with high digitalis doses were noted even in Withering’s day, the effect being described by patients as like looking at the world through a yellowfilter. This yellowing of vision, or xanthopsia, is to remain an unmanageable sideeffect for a long time. What is the root cause? Are the compounds neurotoxic? More so than for many other active ingredients, the adage applies: »The dose makes the poison.«
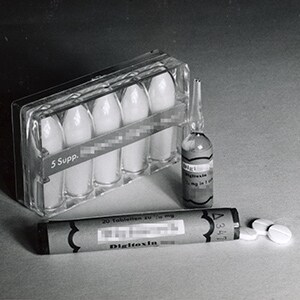
Ultra-high-purity products meeting stringent quality standards are essential in order to accurately adjust the dose to patients – many of whom need the drug to survive– and minimize the risk of xanthopsia. The skills of pharmacologists and formulation experts are required. In 1949, E. Merck, Darmstadt, Germany, launches a special branded Digitoxin medicine. Continuous optimization of bioavailability allows the dose to be kept at the lowest effective level, and the drug becomes an indispensable mainstay of cardiology.
Withering, son of an apothecary, reports having learned the value of digitalis as a treatment for »dropsy« (edema) in 1775 when asked what he thought of a folk herbalist’s homegrown remedy for the condition. Withering systematically investigates the diuretic effect in patients.
»The patient describes the visual phenomenaas follows: flickering, multicolored, irregularly shaped spots. The margins are sharply circumscribed toward the center and are of a dazzlingly bright golden yellow shade. The patient has a blind spot where the visual phenomenon appears. [It is reminiscent of] the color hallucinations experienced on mescaline.«
Experienced in the field of natural substance chemistry, the company recognizes the potential of the digitalis products extracted from the highly toxic foxglove plant intreating congestive heart failure early on, commercializing the first active ingredients in the mid-19th century. In 1949, E. Merck, Darmstadt, Germany, launches »Digimerck« (»Digitoxin Merck«), it becomes an indispensable mainstay of cardiology.